After being accidentally discovered by Brenner and Riddell in the 1940s(1), electroless or autocatalytic nickel has become one of the most important commercial coating technologies used today. This plating process is unique, as it does not require the use of external electrical current to deposit the metal from the solution. Instead, deposits are formed through a chemical reduction-oxidation (or redox) process using specialized reducing agents under specific conditions to transfer electrons between the reacting species. This reaction produces a coating which contains additional element(s) that are deposited into the coating to produce an alloy. Depending on the type and concentration of the alloying species, the coating will exhibit varying properties that differ fundamentally from pure nickel deposits. The most widely utilized type of electroless nickel is electroless nickel-phosphorus (ENP), which is produced using a phosphorus-containing reducing agent, namely sodium hypophosphite. Generally, ENP coatings deliver superior corrosion resistance, improved wear resistance, and higher hardness compared to other plated coatings, along with exceptional deposit uniformity. Since ENP is a chemical process, all surfaces in contact with the plating solution are coated uniformly. This makes ENP particularly valuable on articles that have highly complex geometries.
Historically legislation and regulations have been drivers for innovation in surface finishing technology, and today more sustainable chemistries are required to meet the demands of global regulatory bodies. Additionally, integrating sustainable practices into the development of new technologies is becoming an important selection criterion for future-focused companies. Since the adoption of technologies to meet the ELV Directive 2000/53/EC Annex II(2) in the early 2000s, innovation in ENP has been confined to niche technologies such as poly-alloys, composite coatings, and post-treatment coloring. In recent years, nickel has been a substance of interest to health officials around the world, with nickel compounds classified as human carcinogens(3)(4)(5)(6), so the efficient and responsible use of nickel is key to its continued use. New approaches to electroless nickel are now being examined with this in mind, and the focus on more sustainable approaches is a prominent theme.
The vast majority of conventional ENP processes operate at roughly 6 g/L (0.1 mol/L) Ni metal. A sustainable alternative to this standard is one in which the nickel metal concentration is lowered, thus reducing its impact on both the plating process itself and any downstream operations. With a focus on safety and sustainability, a complete optimization effort was undertaken to create a new class of Reduced Ion (RI) processes that reduce the concentration of nickel metal to roughly 3-4 g/L (0.05-0.07 mol/L).
These efforts resulted in processes that operate at lower Ni metal concentrations while delivering lower solution densities over the solution’s operating lifetime, when compared to conventional chemistries (see Fig 1.)

Figure 1. Solution density comparison for conventional ENP (6 g/L Ni2+) vs RI ENP (3 g/L Ni2+)
As depicted above, the average solution density difference between the RI ENP and the conventional system is roughly 0.025 g/cm3. A MTO (metal turnover), a common measure of ENP bath lifetime, is when the original or make up concentration of nickel metal is depleted from the solution and subsequently replenished. Based on conventional operation the industry has come to define 1 MTO = 6 g/L Ni2+ deposited from solution, so therefore 6 MTO = 48 g/L Ni2+ deposited. It is interesting to note that the equivalent specific gravity of the RI ENP occurs at roughly +1 metal turnover (MTO), meaning the RI ENP gains an additional MTO of solution life compared to conventional EN (the functional end of a bath’s life is often reached when the solution density starts to approach saturation). In addition to the extended operating solution lifetime, other potential advantages of this reduction in solution density include but are not limited to, reduced drag out of EN process solution to post-plate rinses and reduced nickel in emissions from the process solution. An example of said reduction of Ni drag out from the process solution is illustrated in Figure 2.
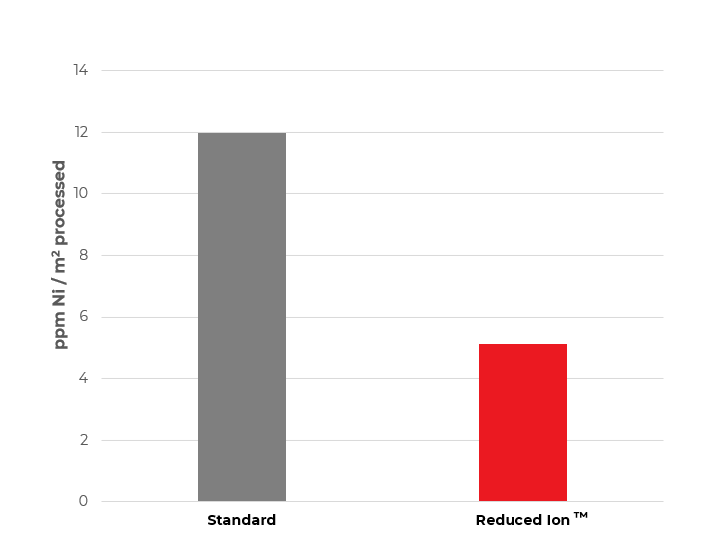
Figure 2. Drag out of nickel metal into first post-plate rinse
Figure 2 depicts the result of an experiment in which 5 consecutive panels (0.015 m2 surface area) were plated in two process solutions at the same stage of solution life (MTO). After plating for 30 minutes, the panels were removed from the process solution and allowed to idle over the solution for 5 seconds before being transferred to a beaker with clean DI water and rinsed thoroughly. The collected rinse water was brought to a standard volume and the Ni concentration was analyzed via atomic absorption spectroscopy. The analysis shows that the RI ENP results in a 55% reduction in Ni metal concentration in the first post-plate rinse, which is a consequence of both the reduced viscosity and lower Ni metal concentration. This effect can be even more pronounced on articles with rougher substrates and/or with more complex geometries.
EN processes operating at lower metal concentrations require less Ni to be stabilized in the solution, resulting in improved plating and operational efficiencies. Stability of an ENP solution is critically important for economical and consistent operation. Since ENP is an autocatalytic reaction, if the process is not sufficiently stabilized it can deposit nickel on the tank walls and any ancillary equipment that the solution comes into contact with. If the solution stability is very poor, the ENP bath could potentially decompose which makes the solution virtually unusable, potentially requiring disposal. Figure 3 illustrates the improved solution stability that RI ENP exhibits compared to a conventional ENP process.
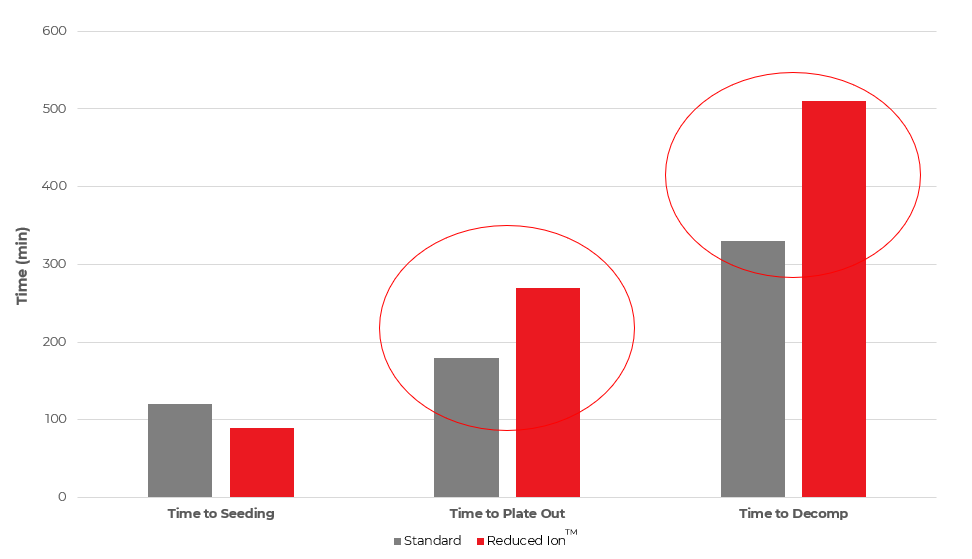
Figure 3. “Boiling stability” of RI ENP vs conventional ENP
The experimental data shown in Figure 3 was generated by boiling a fresh (0 MTO) solution in a clean beaker, free of scratches and etching. The solution volume was maintained at a constant level with DI water throughout the duration of the experiment. The beaker was removed from the heat source and observations regarding the condition of the EN solution were recorded at 30-minute intervals. The critical metrics for evaluation included:
a. Time to initial seeding: Seeding is characterized by visible nickel particles located on the bottom of the beaker. Once seeds form, localized gassing will be observed even after boiling has subsided.
b. Time to plate-out: Plate-out is characterized by the formation of nickel sheets or large nickel solids within the test solution. The plated-out nickel may not adhere to the beaker surface and become free-floating within the solution.
c. Time to decomposition: Decomposition can be characterized in one of two ways:
1. Formation of large black colloidal nickel particles floating within the test solution. A large degree of vigorous gassing often correlates with the onset of decomposition.
2. Some formulations do not achieve full decomposition. In these cases, the plate-out stage continues with the nickel sheets continuing to grow. If this is the case during testing, decomposition would be characterized by nickel concentration decreasing below 15% of the starting nickel concentration.
Figure 3 demonstrates that the RI ENP shows superior solution stability, especially in the case of the
“time to plate-out” and “time to decomposition” metrics, which may directly result in a reduction in plate-out on tanks and ancillary equipment. This improved performance reduces downtime and the labor and chemical costs associated with stripping. At the end of the day, RI ENP improves nickel utilization. This means more nickel is used for value-added plating on customer parts and less time and money is spent on removing nickel from racks.
The adoption of sustainable technology will continue to be a primary area of focus for organizations looking to outpace their competition. As an innovative and sustainable low metal solution, Reduced Ion EN is a next-generation technology that will continue to drive improved performance and efficiency in electroless nickel plating.
Read the full article, available on cfcm.ca
References:
(1) Mallory, G.O. and Hajdu, J. B., (1990) Electroless Plating: Fundamentals and Applications, pp. 3
(2) ELV Directive 2000/53, retrieved from https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32000L0053
(3) International Agency for Research on Cancer (2012). “Nickel and nickel compounds” in IARC Monographs on the Evaluations of Carcinogenic Risks to Humans. Volume 100C. pp. 169–218.
(4) Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC) No 1907/2006 [OJ L 353, 31.12.2008, p. 1]. Annex VI.
(5) Globally Harmonised System of Classification and Labelling of Chemicals (GHS), 5th ed., United Nations, New York and Geneva, 2013.
(6) National Toxicology Program. (2016). “Report on Carcinogens”, 14th ed. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service