Chromium electroplating for functional coatings has been used for decades. These coatings, often referred to as “hard chrome,” have a unique combination of hardness, wear resistance and corrosion resistance that adds critical functionality and value in many applications.
Although useful, chromic acid and other compounds that contain hexavalent chromium, or Cr(VI), ions have long been recognized as highly toxic and carcinogenic. Exposure through inhalation, contact or ingestion poses severe health risks to humans and animals.
Alternative processes have been developed over the years, but none have proven to offer the broad applicability of hard chrome, nor its versatile and cost-effective production characteristics. As a result, none have gained unanimous support.
However, MacDermid Enthone Industrial Solutions has designed a process known as Durati 240 that achieves thick, conformal coatings (more than 100 microns) with wear and fatigue resistance comparable or superior to Cr(VI)-based systems.
‘Hard’ facts about hard chrome
As further proof that a reliable replacement for Cr(VI)-based systems is in demand, it is easy to point to numerous regulations on the process. This includes the U.S. Environmental Protection Agency’s (EPA) Safe Drinking Water Act, Clean Air Act and Resource Conservation and Recovery Act (RCRA), that aim to minimize Cr(VI) exposure. In Europe, Cr(VI) is included in Annex XIV of the REACH Directive, requiring authorization for use. The European Chemicals Agency (ECHA) plans to submit additional restrictions on hexavalent chromium substances under Annex XV. Similarly, China has implemented multiple regulations addressing Cr(VI) in air, water and worker safety standards.
Hard chrome deposits are made from an electroplating solution in which hexavalent chromium often written by its chemical notation Cr(VI) ions are deposited on a part polarized as a cathode. The chromate ions are obtained by combining chromic acid with sulfuric acid and proprietary additives, which enable the deposition of a chromium metal layer alloyed with small amounts of oxygen. The deposited layer does not contain any Cr(VI).
To examine where hard chrome is found in today’s society, it is not “hard” to discover, especially in the automotive industry where hard chrome-plated parts are used for shock absorbers, pistons and engine valves. The construction industry uses hydraulic rods in mining machinery and other heavy equipment, and every one of those hydraulic cylinders has a hard chrome coating. Aerospace and military sectors also favor hard chrome because of its durability and ability to perform reliably under extreme conditions, leading to its use on aircraft landing gear and door hooks.
However, it is important to distinguish between hard chrome and chrome coating used as a decorative trim on cars and consumer goods. Decorative chrome is a complex multilayer coating of copper and nickel topped with a thin final layer of chromium and is not considered harmful.
PFAS problems come into play
In addition to Cr(VI) exposure concerns, the electrolytes in hard chrome plating often contain additives that are under scrutiny. Per- and polyfluorinated Alkyl Substances (PFAS) are often used as mist suppressants in hard chrome plating operations. Although these substances reduce toxic mists around the plating baths by lowering the surface tension and preventing bubble formation, PFAS have their own set of problems.
PFAS contain fully and partly fluorinated carbon chains of various lengths attached to a functional group such as a carboxylic or sulphonic acid1. The carbon-fluorine bond is strong, and PFAS are among the few chemicals that can survive in the highly oxidizing environment of a chromic acid-based electrolyte.
However, this chemical durability is also the source of environmental concern, as PFAS are persistent and bio-accumulate. Their persistence in soil, water and living organisms has earned them the nickname “forever chemicals.” This has led to significant regulatory attention, particularly in the U.S. In Europe, the ECHA proposed restricting more than 10,000 PFAS substances in February 20232.
Given the environmental and health risks that Cr(VI) and PFAS bring, it is clear that the current hard chrome production process is problematic.
Hard chrome properties
Before evaluating alternatives, it is important to understand the properties of hard chrome coatings. Traditional Cr(VI)-based hard chrome technology involves plating a relatively thick layer of hard chrome, (for example, 25 microns), which will deliver a hardness of 1,000 HV0.1. This layer may also need to be machined to have the fine dimensional tolerances required in the demanding mechanical systems where these parts are used. Cr(VI)-based coatings can withstand this machining without compromising their properties. Although not required, the coating can also tolerate heat treatment if needed to distress the underlying substrate for hydrogen release or tempering.
The result is a thick, uniform layer of a highly wear-resistant surface that is also able to retain sufficient lubrication to achieve a smooth ride on your car suspension or to operate hydraulics for long periods without oil leakage. The useful lifetime of shocks in many cars is close to 100,000 miles, all while housed in a wheel well that sees water, corrosive road salts, abrasive particles and huge temperature swings. And that’s a pleasant environment compared to those experienced by hydraulics used in construction or military equipment.
A useful metaphor for understanding the challenge of finding alternatives is that of an iceberg. Some properties of hard chrome are immediately visible and well understood, but many others lie beneath the surface, unspoken yet essential to performance.
Alternatives to hexavalent hard chrome
The search for alternatives to Cr(VI)-based hard chrome plating has been ongoing for several years, with various technologies proposed as replacements. These ideas have included plasma-applied metallic alloys, electrodeposition of alloys from less toxic precursors, electroless nickel plating with hard inclusions and a range of metallurgical treatments. However, MacDermid’s Duratri 240 process developed in partnership with IRT M2P is especially promising.
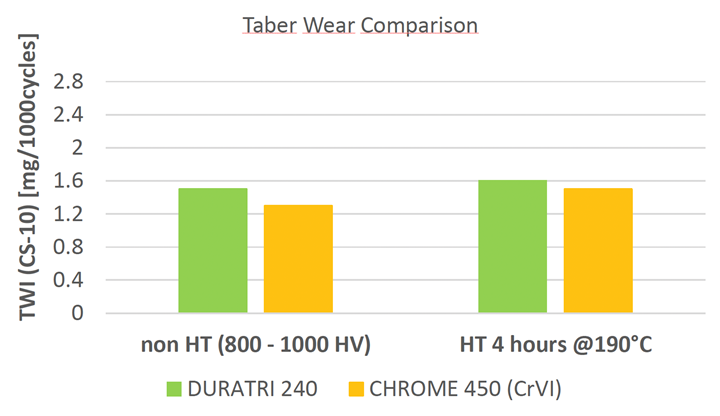
Not only does the process achieve thick, conformal coatings (more than 100 microns) with wear and fatigue resistance comparable or superior to Cr(VI)-based systems, it is developed with sustainability in mind. It avoids regulated substances, including boric acid and PFAS. While this development represents a significant advancement, challenges remain.
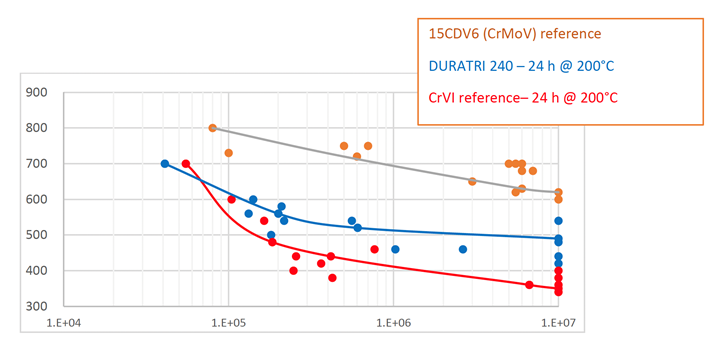
Obstacles surrounding the new process are caused by the fundamental differences between Cr(VI) and Cr(III) electrolytes. Cr(III) systems tend to produce an amorphous chromium-carbon-oxygen alloy, rather than the (110) crystalline structure typical of Cr(VI). This leads to different behavior in heat treatment, as Cr(III)-based hard chrome layers tend to increase in hardness. Although this is not necessarily a problem, it is a difference one must be aware of. The amorphous alloy is more prone to through-cracking after mechanical or thermal treatments, compromising corrosion resistance. While this can be addressed by adding a nickel underlayer, ongoing testing continues to improve these processes for different applications.
Developments and perspectives
MacDermid Enthone’s experience to date has informed ongoing development efforts. Recognizing that the best path to acceptance in the industry is a process that most closely replicates current versions of hard chrome, they are focused on duplicating a chromium deposit with the preferred (110) crystal orientation.
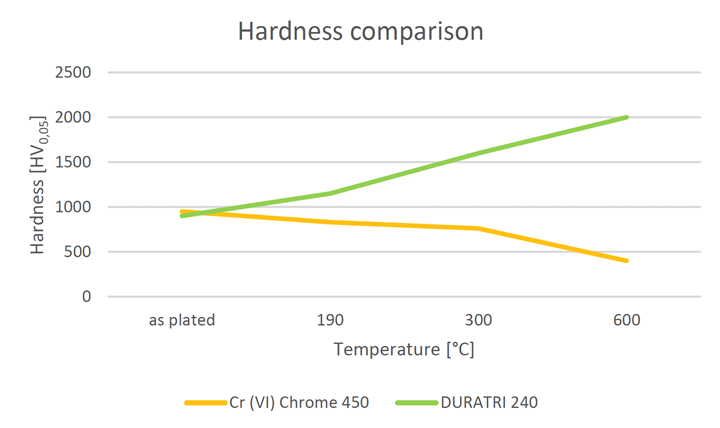
Some success has been seen on copper-based substrates and is currently being developed for steel substrates. Initial results on piston rod samples plated by one of these development processes after grinding showed a structure free of through-cracks and a hardness that meets the requirements. This system currently necessitates the use of a specific reactor cell to accurately monitor and control the plating parameters. Although this poses some challenges, it has provided encouraging results.
MacDermid Enthone has partnered with IRT M2P on the new Neptune project, as a continuation of the two previous projects, to develop an alternative to functional hard chromium, known as HCTC and Cronos. 
One of the goals of the project is to develop a carbon-free electrolyte to avoid the incorporation of carbon into the chromium deposit for the most critical applications such as aerospace and military. The increased hardness and the formation of through cracks after machining or heat treatment is not acceptable for such applications.
Preliminary tests have shown that it is possible to obtain a carbon-free deposit consisting mainly of chromium and oxygen from a trivalent chromium electrolyte. The resulting properties are being investigated, and the process will continue to be developed.
The elimination of Cr(VI) from the hard chrome production process is a tall order, but the benefits to industry and society are enormous. The technical objective is to duplicate the (110) crystalline structure of hard chrome made from Cr(VI). This will make the coating properties similar and enhance the speed adoption of new production processes. Trivalent systems, including the Duratri range, are proving that high-performance coatings can be achieved without relying on hexavalent chromium. Our challenge is to expand these early demonstrations of feasibility into robust, economical manufacturing processes that will make a shift to Cr(III)-based hard chrome acceptable to the entire supply chain for this critical coating.
References
(1) eur-lex.europa.eu/legal-content/EN/ALL/uri=SWD%3A2020%3A249%3AFIN
(2) echa.europa.eu/-/echa-publishes-pfas-restriction-proposal
(3) Zeng, A review of recent patents on trivalent chromium plating. Recent Patents on Materials Science. 2009, 2, 50-57
Read the full article, available now at pfonline.com.